Removing soluble impurities
Key Notes:
What Are Soluble Impurities?
- Soluble impurities are substances that can dissolve in a liquid, like salt in water.
Why Remove Soluble Impurities?
- We want to remove soluble impurities to make the liquid cleaner and safer to use or drink.
- Filtration
- Filtration is a method to remove solid impurities from a liquid.
- It uses a filter or a sieve to trap the solid particles while letting the liquid pass through.
- Example: Making Tea
- When making tea, we use a tea strainer to remove tea leaves (soluble impurities) from the tea.
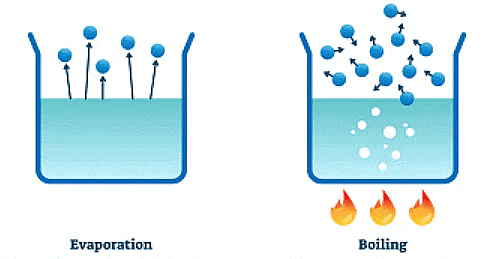
- Evaporation
- Evaporation is a way to remove the liquid from a solution and leave the solid impurities behind.
- For example, if you have a salty solution, you can heat it to make the water evaporate, leaving the salt behind.
- Distillation
- Distillation is a method to separate different liquids in a mixture based on their boiling points.
- This is used to purify water by boiling it and collecting the steam, which leaves impurities behind.
- Crystallization
- Crystallization is a process where we let a solution cool and form crystals.
- The crystals are pure, and we can separate them from the impurities.
- Using Tools
- We can use tools like filters, sieves, strainers, and heating equipment to remove soluble impurities.
- Safety
- Always be safe when handling chemicals or hot substances. Use adult supervision when needed.
- Everyday Examples
- Removing sand from water at the beach.
- Making sugar crystals from a sugar-water solution.
Let’s practice!

